Hematogones
You are here
Definition
Hematogones are benign lymphoid precursors whose morphology and immunophenotype are similar to the blasts found in ALL. These cells are more commonly found in pediatric bone marrow aspirates following therapy. The clinical significance of expanded HGs is unknown, and the biologic features of these cells are still incomplete.
Sample Cases
Click here for instructions on how to download the free FCS Express Reader to view and manipulate the sample cases.
| Case Name (click on case name to open) |
Comments | Size |
| case 11 |
hematogones This case was kindly provided by the ASCP Press. It is part of Flow Cytometry in Clinical Diagnosis by John Carey, Phil McCoy and David Keren. |
1939 kB |
| BM Aspirate UTMC | "Normal" Bone Marrow aspirate submitted by UTMC for reference with their submitted wiki cases. | 3.5 Mb |
Epidemiology
Hematogones are found in highest numbers in bone marrow of infants and young children, with a statistically significant decrease in their percentage with increasing age.
Possible causes
The clinical conditions in which 5% or more of more hematogones are found are listed in table below; these include lymphomas, various non-neoplastic cytopenias, post-chemotherapy, post-bone marrow transplant,and AIDS were the most common.
|
N = 53 with 5% or more bone marrow hematogones |
|
|
|
|
| Clinical condition | Number of specimens |
|
|
|
| Hodgkin lymphoma following treatment | 2 |
| De novo non-Hodgkin lymphoma | 11 |
| Pretreatment | 8 |
| Posttreatment | 3 |
| Non-Hodgkin lymphoma with AIDS | 2 |
| Pretreatment | 1 |
| Posttreatment | 1 |
| HIV/AIDS without lymphoma | 5 |
| Post-chemotherapy for ALL or AML (8 patients) | 9 |
| Post-bone marrow transplantation for AML or MDS | 5 |
| Pretreatment AML (1 patient) | 2 |
| Various nonneoplastic blood cytopenias | 14 |
| Pancytopenia | 4 |
| Neutropenia | 6 |
| Thrombocytopenia | 4 |
| Reactive lymphocytosis | 1 |
| Neuroblastoma | 1 |
| Other (cough, weight loss) | 1
|
| Total specimens | 53 |
| Total patients with NHL | 13 |
| Total patients with HIV/AIDS | 7 |
| Total patients post-chemotherapy | 14 |
|
|
|
Morphology
Morphologically hematogones are distinct lymphoid cells with condensed and homogeneous chromatin, and scant cytoplasm. These cells can be observed in large numbers in the bone marrow of children with a variety of hematologic and nonhematologic disorders. They varied from 10 to 20 µ in diameter, with smaller cells predominating. The nucleus is round or oval and can exhibit one or more indentations or shallow clefts. Nucleoli were absent or small and indistinct. There is generally scant or no discernible cytoplasm; when present, cytoplasm is moderately to deeply basophilic and devoid of granules, inclusions, or vacuoles. There is often a variety of size and cytologic features that blends with those of mature lymphocytes. Frequently a portion of the hematogones exhibited features indistinguishable from lymphoblasts of ALL.
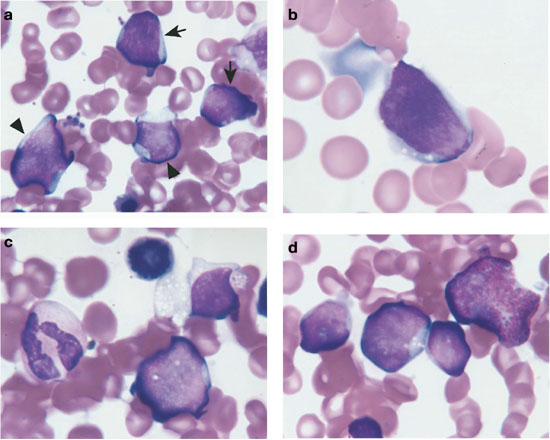 |
| Example morphology of HGs |
Immunophenotyping
Hematogones have a characteristic profile of CD38++, CD10+, D19+, sIg-, Fc receptor negative, CD20- or dim, and a cluster often found dimmer and smaller on the CD45/log side scatter display. The CD10, CD38, CD45 combination can be useful. The goal is to distinguish ALL from normal precusor B cells. For detection at a low level (<2%) it is useful to know the original antigenic fingerprint of the ALL clone.
Flow cytometric analyses of bone marrow cells demonstrates a spectrum beginning from early B-cell precursors (CD10+,CD19+, TdT+, HLA-Dr+) to mature sIg-bearing B cells in these patients, intermediate forms, and mature lymphocytes. DNA content is normal, and no clonal abnormality is identified by either cytogenetic or immunoglobulin and T-cell receptor (TCR) gene rearrangement studies.
The earliest recognizable B-lineage precursors expresses CD34 in combination with CD38, CD19, high levels of CD10(bright), and low levels of CD22 and lacking CD20. These cells progress to the next stages by down-regulating CD34 completely and CD10 partially, prior to the progressive up-regulation of CD20. CD22 levels are also increased slightly as CD20 is up-regulated. Finally, CD10 is down-regulated completely, CD38 partially, and CD22 upgraded to high intensity.The last stage, in which CD10 is completely down-regulated, is considered a mature stage of B-cell ; cells with this immunophenotype were not included in calculating the percent hematogones. In addition, TdT expression parallels CD34 in the B-cell maturation sequence. Asynchronous expression of the earliest and latest antigens, eg, concurrent CD34 and CD20,and aberrant over- or under-expression of antigens was not observed in hematogone populations.
Other relevant tests
Cytochemistry
Genetics
Sub-classification
The antigen expression of normal B lymphoid cells is heterogeneous but with a distinct relationship between antigens. The combination of antigens change during normal development and can be used to define 4 stages which are quite
distinct(3,5). These relationships are maintained from fetal development to the elderly and following chemotherapy and bone marrow transplantation(4). The phenotypes of B-ALL do not fit into these stages and therefore can be used to identify the leukemic cells based on their aberrancy of antigen expression(6). Therefore, hematogones follow normal development while residual ALL have abnormal phenotypes which can be distinguished down to 0.1% post therapy(7).
Flow Diagnosis
Hematogones are caraterized by very low side scatter (SSC) and dimmer expression of CD45 when compared to lymphocytes. They have variable ('smeared') expression of CD20; are positive for CD10 and partially for CD34 (B).
References
1. http://bloodjournal.hematologylibrary.org/cgi/content/full/98/8/2498#T4
2. Hassanein NM, Alcancia F, Perkinson K, Buckley P, Lagoo A. Distinct Expression Patterns of CD123 and CD34 on Normal Bone Marrow B-Cell Precursors (“Hematogones”) and B Lymphoblastic Leukemia Blasts (2009) American Journal of Clinical Pathology, 132, 573-580
3. Quantitative flow cytometry can distinguish between normal and leukaemic B-cell precursosrs.
N. Farahat et al. British Journal of Haematology, 1995, 91, 640-646
4. Loken et al. Blood 70:528-531, 1988
5. LeBien et al. Leukemia 4: 354-358, 1990
6. Ryan et al. Blood 68:417-425, 1986
7. Hurwitz et al. Blood 72:299-307, 1988
8. Wells et al. Blood 86: 3132 (Supp1), 1995