Myelodysplastic Syndromes (MDS)
You are here
Definition
The myelodysplastic syndromes (MDS, formerly known as "preleukemia") are a diverse collection of hematological conditions united by ineffective production of blood cells and varying risks of transformation to acute myelogenous leukemia (AML).
Myelodysplastic syndromes (MDS) are bone marrow stem cell disorders resulting in disorderly and ineffective hematopoiesis (blood production) manifested by irreversible quantitative and qualitative defects in hematopoietic (blood-forming) cells. In a majority of cases, the course of disease is chronic with gradually worsening cytopenias due to progressive bone marrow failure. Approximately one-third of patients with MDS progress to AML within months to a few years.
Sample Cases
Click here for instructions on how to download the free FCS Express Reader to view and manipulate the sample cases.
| Case Name (click on case name to open) |
Comments | Size |
| case 24 |
Agressive MDS - Refractory Cytopenia w/ multilineage dysplasia This case was kindly provided by the ASCP Press. It is part of Flow Cytometry in Clinical Diagnosis by John Carey, Phil McCoy and David Keren. |
4.7 mB |
Epidemiology
The median age at diagnosis of a MDS is between 60 and 75 years; a few patients are less than 50; MDS are rare in children. Males are slightly more commonly affected than females. Signs and symptoms are nonspecific and generally related to the blood cytopenias. Some estimates are on the order of 10,000 to 20,000 new cases each year in the United States alone. The incidence is probably increasing as the age of the population increases, and some authors propose that the incidence in patients over 70 may be as high as 15 cases per 100,000 per year.
Although there is some risk for developing acute myelogenous leukemia, about 50% of deaths occur as a result of bleeding or infection. Leukemia that occurs as a result of myelodysplasia is notoriously resistant to treatment.
Possible causes
MDS is caused by environmental exposures such as radiation and benzene; other risk factors have been reported inconsistently. Secondary MDS occurs as a late toxicity of cancer treatment, usually with a combination of radiation and the radiomimetic alkylating agents such as busulfan, nitrosourea, or procarbazine (with a latent period of 5 to 7 years) or the DNA topoisomerase inhibitors (2 years). Both acquired aplastic anemia following immunosuppressive treatment and Fanconi's anemia can evolve into MDS.
Morphology
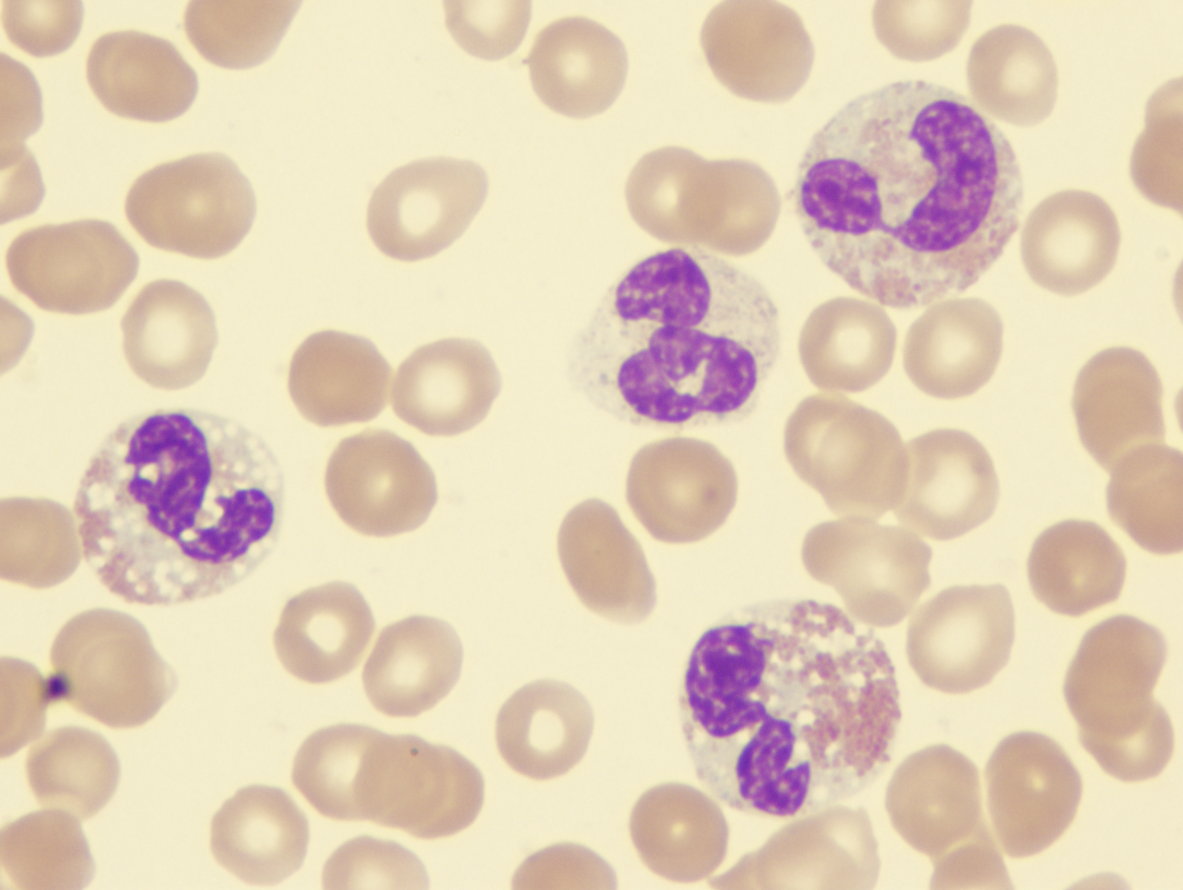
Dysplasia can affect all three lineages seen in the bone marrow. The best way to diagnose dysplasia is by morphology and special stains (PAS) used on the bone marrow aspirate and peripheral blood smear. Dysplasia in the myeloid series is defined by:
- Granulocytic series
- Hypersegmented neutrophils (also seen in Vit B12/Folate deficiency)
- Hyposegmented neutrophils (Pseudo-Pelger Huet)
- Hypogranular neutrophils or pseudo Chediak Higashi large granules
- Dimorphic granules (basophilic and eosinophilic granules) within eosinophils
- Erythroid series
- Binucleated erythroid percursors and karyorrhexis
- Erythroid nuclear budding
- Erythroid nuclear strings or internuclear bridging (also seen in congenital dyserythropoietic anemias)
- PAS (globular in vacuoles or diffuse cytoplasmic staining) within erythroid precursors in the bone marrow aspirate (has no bearing on paraffin fixed bone marrow biopsy). Note: One can see PAS vacuolar positivity in L1 and L2 blasts (AFB classification; the L1 and L2 nomenclature is not used in the WHO classification)
- Ringed sideroblasts seen on Prussian blue iron stain (10 or more iron granules encircling 1/3 or more of the nucleus and >15% ringed sideroblasts when counted amongst red cell precursors)
- Megakaryocytic series (can be the most subjective)
- Hyposegmented nuclear features in platelet producing megakaryocytes (lack of lobation)
- Hypersegmented (osteoclastic appearing) megakaryocytes
- Ballooning of the platelets (seen with interference contrast microscopy)
 |
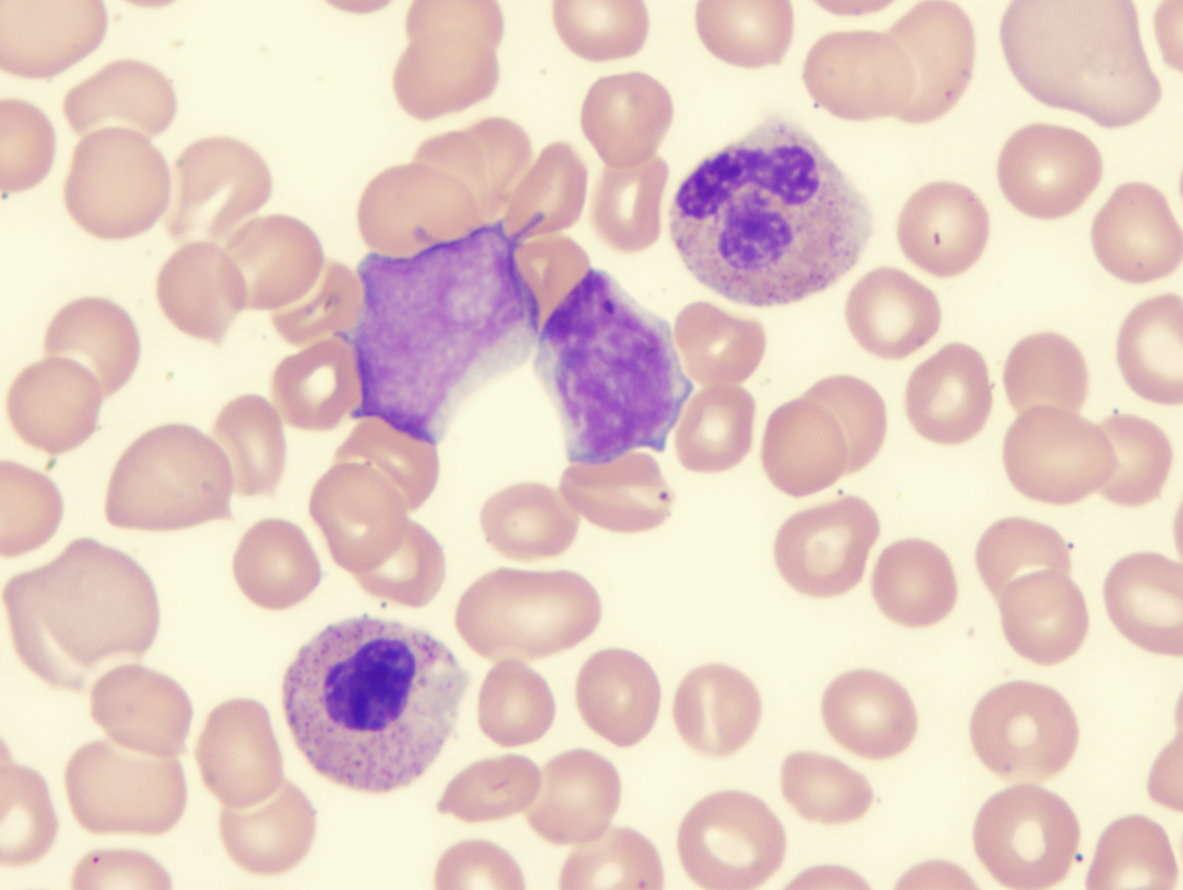 |
| Myelodysplastic-syndrome-dysplastic-eosinophils-demonstrating-abnormal-lobation-and-hypogranulation | Myelodysplastic-syndrome-showing-blast-and-monolobed-neutrophil |
Immunophenotyping
Antigenic abnormalities have also been identified by flow cytometry in MDS patients, compared to normal controls. These abnormalities have included (1) loss of erythrocyte A, B, and H antigens in MDS; (2) decreased expression of c-Mpl, GPIIb/IIIa, and GPIb on platelets from patients with refractory anemia; (3) dyssynchronous expression of CD11b and CD16 in the developing neutrophils of patients with MDS; (4) decreased CD10 on neutrophils in MDS; (5) changes in a variety of leukocyte activation antigens, including FcRI, FcRII, and FcRIII, in MDS; (6) greater variability in the expression of CD38, CD71, CD13, and CD33 in refractory anemia versus either normal marrow or marrows involved by aplastic anemia; and (7) aberrant coexpression of CD56 on myeloid blasts in MDS. (1)
Other relevant tests
Cytochemistry
Other stains can help in special cases (PAS and napthol ASD chloroacetate esterase positivity) in eosinophils is a marker of abnormality seen in chronic eosinophilic leukemia and is a sign of aberrancy.
Genetics
Sub-classification
The list of dysplastic syndromes under the new WHO system includes:
- Refractory anemia (RA)
- Refractory anemia with ringed sideroblasts (RARS)
- Refractory cytopenia with multilineage dysplasia (RCMD)
- Refractory cytopenia with multilineage dysplasia and ringed sideroblasts (RCMD-RS)
- Refractory anemia with excess blasts I and II
- 5q- syndrome
- Myelodysplasia unclassifiable (seen in those cases of megakaryocyte dysplasia with fibrosis and others)
Flow Diagnosis
References
1. Stetler-Stevenson, M., D.C. Arthur, N. Jabbour, et al., Diagnostic utility of flow cytometric immunophenotyping in myelodysplastic syndrome. Blood, 2001. 98: p. 379-387.
2. Kussick, S., J.R. Fromm, A. Rossini, et al., Four-color flow cytometry show strong concordance with bone marrow morphology and cytogenetics in the evaluation for myelodysplasia. American Journal of Clinical Pathology, 2005. 124: p. 170-181.
3. Malcovati, I., M.G. Della Porta, M. Lunghi, et al., Flow cytometry evaluation of erythroid and myeloid dysplasia in patients with myelodysplastic syndrome. Leukemia, 2005. 19: p. 776-783.
4. Matsui, W.H., R.A. Brodsky, B.D. Smith, et al., Quantitative analysis of bone marrow CD34 cells in aplastic anemia and hypoplastic myelodysplastic syndromes. Leukemia, 2006. 20: p. 458-462.
5. Xu, Y., R.W. McKenna, N.J. Karandikar, et al., Flow cytometric analysis of monocytes as a tool for distinguishing chronic myelomonocytic leukemia from reactive monocytosis. American Journal of Clinical Pathology, 2005. 124: p. 798-806.
6. Brunning, R.D., J. Bennett, G. Flandrin, et al., Refractory cytopenia with multilineage dysplasia, in Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues, E.S. Jaffe, et al., Editors. 2001, IARC Press: Lyon, France. p. 70.
7. Kussick, S., Wood, B., Using 4-Color Flow Cytometry to Identify Abnormal Myeloid Populations. Archives of Pathology and Laboratory Medicine: Vol. 127, No. 9, pp.1140-1147.