Acute B Lymphoblastic Leukemia (B-ALL)
You are here
Definition
Acute lymphoblastic leukemia (ALL) is a clonal expansion of the lymphoid blasts in bone marrow, blood or other tissues. ALL can be either T or B lineage (see T ALL) . Pre-B ALL is the proliferation of the blasts of the B lineage.
Sample Cases
Click here for instructions on how to download the free FCS Express Reader to view and manipulate the sample cases.
| Case Name (click on case name to open) |
Comments | Size |
| ALL-1 |
pre-B All |
5 Mb |
| ALL-2 |
pre-B ALL with myeloid Antigens |
7 Mb |
| ALL-3 |
pre B ALL |
2.86 Mb |
| case 22 | pre B ALL This case was kindly provided by the ASCP Press. It is part of Flow Cytometry in Clinical Diagnosis by John Carey, Phil McCoy and David Keren. |
7.54 MB |
| ALL case | pre B ALL case submitted by UTMC. Compare with Normal PB. | 2.8 MB |
Epidemiology
The incidence of acute leukemia is approximately 4 cases per 100,000 per year. 30% of these are ALL. ALL is generally seen in children 75% of cases are usually in children under 6 years of age. There are approximately 3200 cases of ALL in the United States per year; 80-85% of these are precursor B-ALL, the others are precursor T-ALL. The overall cure rate in children is 85%, and about 50% of adults have long-term disease-free survival.
Possible causes
Similar to AML, possible factors that have been associated with ALL are viruses, radiation, cytotoxic chemotherapy and benzene exposure. Exposure to the atomic bomb of WWII increased the incidence of all leukemias including ALL. There is also evidence that may suggest a genetic factor in some cases.
Morphology
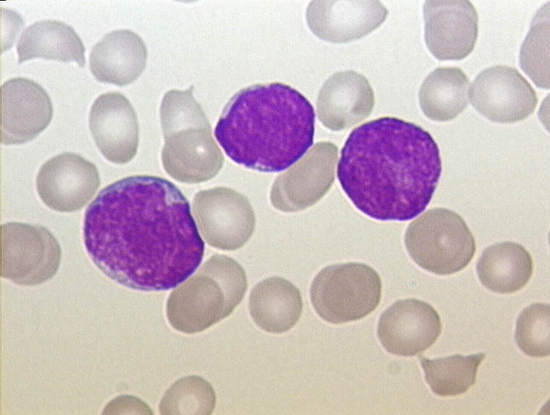
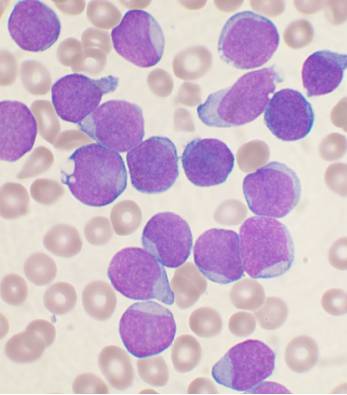
Lymphoblasts (T or B) vary in size from similar to that of a mature lymphocyte to the size of a neutrophil. These blasts have scant to moderate basophillic cytoplasm with occassional azurophillic granules (T ALL). The nuclear chromatin can be fine to clumped, with occasionally prominant nucleoli.
 |
|
Typical morphology of B lymphoblasts
WHO classification
The recent WHO International panel on ALL recommends that the FAB morphologic classification (L1, L2, L3) be abandoned, since this classification has no clinical or prognostic relevance. It instead advocates the use of the immunophenotypic classification. There are 2 main immunologic types: pre-B cell and pre-T cell. The mature B-cell ALL (L3) is now classified as Burkitt leukemia/lymphoma. Subtyping helps determine the prognosis and most appropriate treatment in treating ALL.
Immunophenotyping
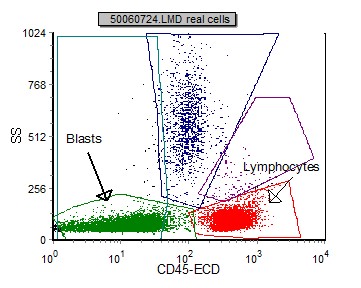
Blasts in pre B ALL can be initially identified using a SSC vs CD45 plot. These blasts have low SSC (many times smaller than normal lymphocytes) and dim to negative CD45. This differs from blasts in AML where the SSC is generally higher.
 |
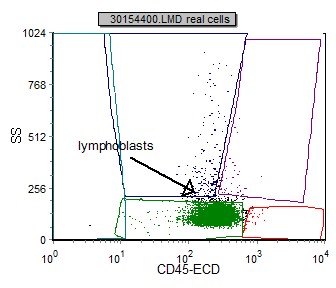 |
| Example peripheral blood with CD45 negative B lymphoblasts and characteristically small (low SSC) | Example bone marrow with CD45 dim B lymphoblasts. |
Once the blasts are identified and gated, the following markers are useful in classification of pre B ALL. Included are marking prevalence:
| Marker | Prevalence |
| CD10 | 89% |
| CD13 | 5% |
| CD19 | 100% |
| CD20 | 24% |
| CD22 | 69% |
| CD33 | 31% |
| CD34 | 76% |
| CD45 (bright) | 2% |
| CD45 (moderate) | 33% |
| CD45 (dim) | 36% |
| CD45 (negative) | 29% |
| CD56 | 36% |
| CD79a | 88% |
| CD117 | 0% |
| cytoplasmic IgM | 22% |
| HLA Dr | 98% |
| TdT | 91% |
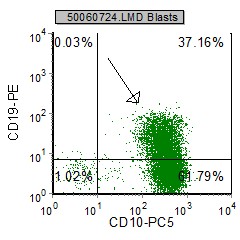
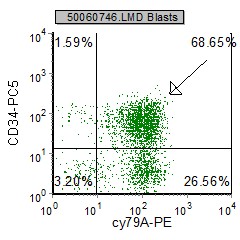
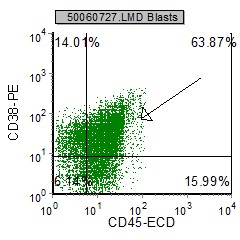
Example Dot plots in pre B ALL
 |
 |
 |
 |
 |
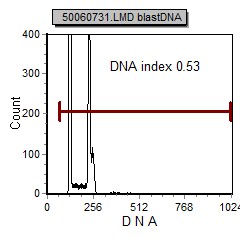 |
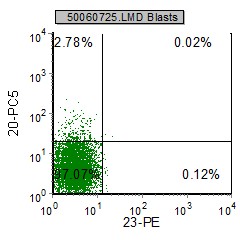
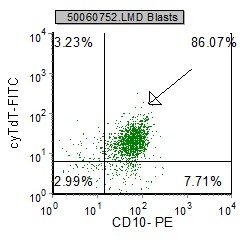
| pre B ALL cells characteristically coexpress CD10 and CD19. In addition, CD20 and CD23 (other B cell markers) are not expressed. | Expression of CD34 and TdT indicates immaturity and is characteristic in pre B ALL. cytoplasmic expression of CD79 confirms B cell lineage. | CD38 is an expression of immaturity. The ploidy as seen by the DNA Histogram, indicates a hypodiploidy (DI=0.53) indicating poor prognosis. |
The phenotype of the blasts is an idependent prognostic parameter. B-ALL is subdivided into:
early pre B-ALL: TdT+, CD19+, CD10-
common ALL: CD19+, CD10+/CALLA+
pre B ALL: CD10+/-, CD19+, HLA Dr+, cytoplasmic IgM+
mature B ALL: CD10+, CD19+, CD20+, CD22+, surface IgM+
Other relevant tests
Genetics: Some cytogenetic subtypes have a worse prognosis than others. These include:
- A translocation between chromosomes 9 and 22, known as the Philadelphia chromosome, occurs in about 20% of adult and 5% in pediatric cases of ALL.
- A translocation between chromosomes 4 and 11 occurs in about 4% of cases and is most common in infants under 12 months.
- Not all translocations of chromosomes carry a poorer prognosis. Some translocations are relatively favorable. For example, Hyperdiploidy (>50 chromosomes) is a good prognostic factor.
Flow Diagnosis
B ALL: CD45 downregulated, CD19+, TdT+, CD10+, surface light chains negative.
References
World Health Organization Classification of Tumors. Tumors of Haematopoetic and Lymphoid Tissue. Jaffe, E., Harriss, N., Stein, H., Vardiman, J. IARC Press 2001
Flow Cytometry in Neoplastic Hematology-Morphologic-Immunophenotypic Correlation. Gorczyca W., Informa Healthcare 2007
Clinical Flow Cytometric Analysis of Hematolymphoid Cells; Approved Guideline - Second Edition H43-A2 Clinical and Laboratory Standards Institute (CLSI) 2007